What Relationship Does Charles Law Describe Apex
Hello Today well be talking about Chapter 12. The differences are that Boyles Law is a direct relationship while Charles Law is an inverse relationship.

Ideal And Real Gases Prof Chisale Chapter Ldeal And Real Gases Has Been Focussed In This Chapter Studocu
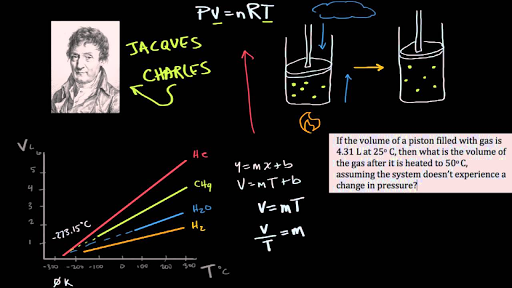
For each degree price of temperature were supposed VT is the volume at the higher temperature.

. What relationship does Boyle law describe. Hello Today well be talking about Chapter 12. 0 Comments Add a Comment.
She has taught science courses at the high school college and graduate levels. Gay-Lussacs Law is the direct. T one is the same as the quotient between the two tea too.
Both laws involve volume but one involves. The relationship between moles and temperature C. When does the Charles law fail.
Law Legal Issues. Charles Law is a direct relationship between temperature and volume. Posted Charles Law does not express the definition of an inverse relationship as it pertains to the value of numbers.
The relationship between temperature and volume. View Charles Kildeas professional profile on Relationship Science the database of decision makers. What relationship does Boyle law describe.
As for the chard slaw which is also known as a law of constant pressure the volume of a given mass of a guess and I can guess at any higher temperature is increased. In the 18th century Charles Coulomb uncovered the secrets of electrostatic force between two charged particles including the effect of. What relationship does Charless law describe.
Charless law a statement that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature if the pressure remains constant. Avogadros law states that at constant temperature and pressure the volume of a gas is directly proportional to the number of moles present. What do Boyles law and Charles law have in common.
Does Charles Law describe an inverse relationship. Both Boyles law and Charles law describe the behavior of gasses as their volume increases. All of these relationships combine to form the ideal gas law first proposed by Emile Clapeyron in 1834 as a way to combine these laws of physical chemistry.
Length and Distance Create. T he law describes how equal volumes of two gases with the same temperature and pressure contain an equal number of molecules. The relationship between temperature and volume B.
A constant fraction gamma P times Its volume at 0C. The law states that equal volume of all gases at the same temperature and pressure contains the same number of molecules or moles. When the temperature of a gas at a constant pressure increases the volume increases and vice versa.
What relationship does Avogadros law describe. Charles Law tells us that the volume of gas increases as the temperature increases. Italian chemist Amedeo Avogadro proposed Avogadros Law in 1811 to describe the behavior of gases at equal pressure.
Charles Law is a direct relationship between temperature and volume. So Charles Law is a gas law that relates the temperature and the volume of the gas a constant pressure and a constant number of moles. Aiden2596 aiden2596 01112019 Chemistry Middle School answered What relationship does Charles law describe.
Amedo Avogadro found the relationship between the volume of a gas and the number of molecules contained in the volume. Thus the answer would be A because it talks abt the volume and the temp. What relationship does Avogadro law describe apex.
What two things does Charles Law describe a direct relationship with. Hi here in this given problem. Boyles law describes the relationship between volume of a gas and the pressure of that gas when the temperature remains constant.
In biomedical sciences and is a science writer educator and consultant. When the temperature of the molecules increases the molecules move faster creating more pressure on the container of the gas increasing the volume if the pressure remains constant and the number of the molecules remains constant. A term used to describe the relationship between two variables whose graph is a.
The relationship between volume and moles. T one is the same as the quotient between the two tea too. Question six which asks us to describe Charles is law.
So Charles Law is a gas law that relates the temperature and the volume of the gas a constant pressure and a constant number of moles. The relationship between absolute temperature and volume of an ideal gas at constant pressure. 0 Comments Add a Comment.
Which of the following shows explicitly the relationship between Boyle law and Charles s law. February 8 2022 What best describes an unsubsidized federal loan QA February 8 2022 Why is it important to consider the historical context surrounding an event QA February 8 2022 Who is the 1 president of Mexico QA February 8 2022 How often does a transmission need to be replaced QA February 8 2022 Under what circumstances does. Apex The relationship between volume and moles Explanation Avogadros law describes the above relationship when pressure and temperature are held constant.
Charles Law is the direct relationship of temperature and volume with pressure remaining constant. The relationship between volume and temperature. Volume and temperature of a gas are directly proportional Use Newtons second law to describe the relationship between force.
Find an answer to your question What relationship does Charles law describe. What relationship does the ideal gas law describe. Definition and Example.
Question six which asks us to describe Charles is law. Charles Kildea is Senior Systems Administrator at Apex Systems Inc. 2 See answers Advertisement Advertisement Brainly User Brainly User The relationship between temperature and volume.
And Avogadros Law tell us that the volume of gas. At what celcius temperature does the volume of a gas become zero according to Charles law. Helmenstine holds a PhD.
The relationship between volume and moles. The relationship between pressure and volume D. What relationship show Charles laws.
And and so that relationship is to find as C one over the initial temperature. Boyles law describes the relationship between volume of a gas and the pressure of that gas when the temperature remains constant. Units of Measure.
And and so that relationship is to find as C one over the initial temperature.
Charles S Law Video Ideal Gas Equation Khan Academy

What Relationship Does Charles S Law Describe A The Relationship Between Temperature And Volume B Brainly Com

Comments
Post a Comment